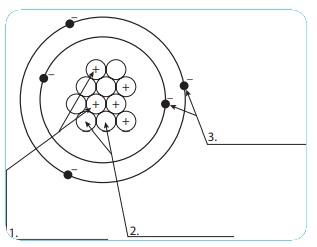
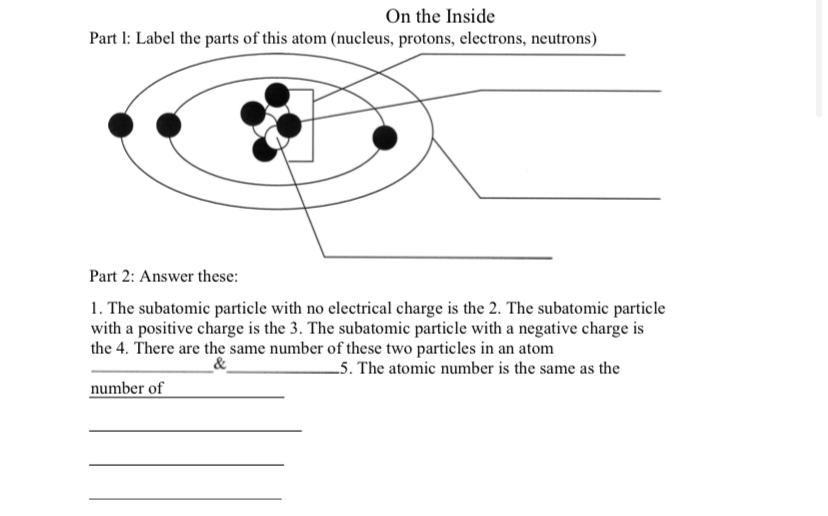
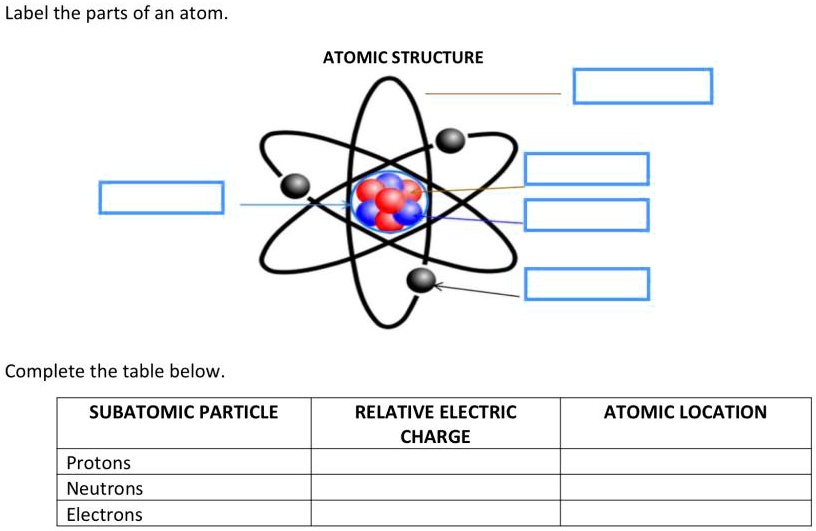
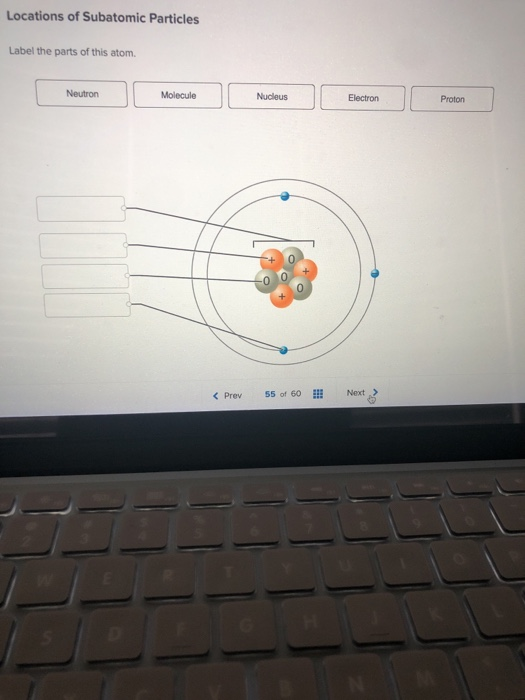
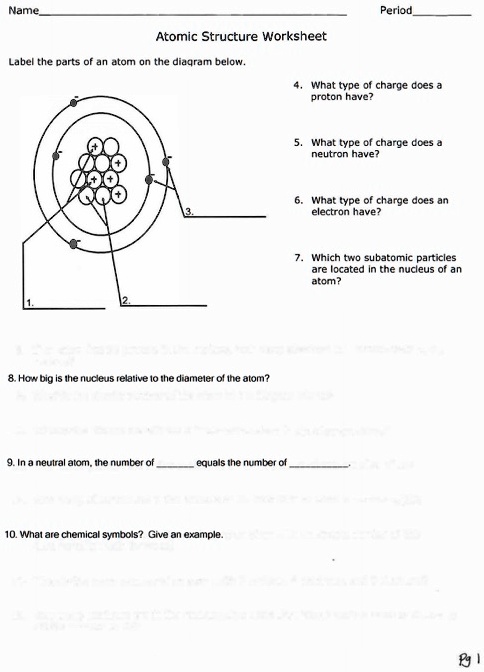
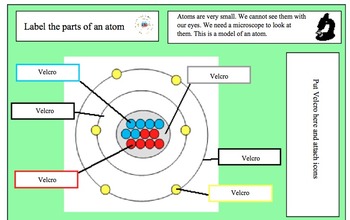
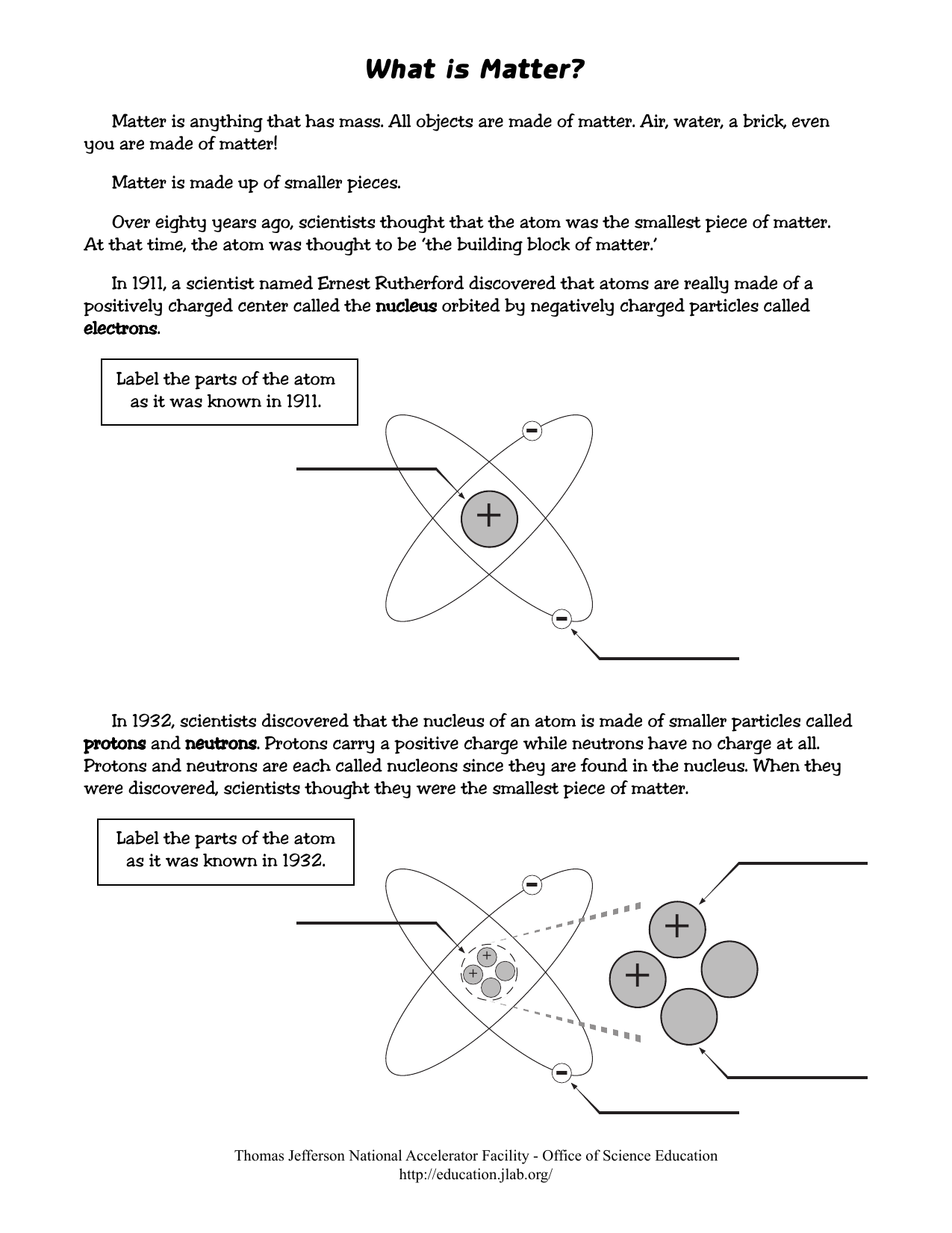
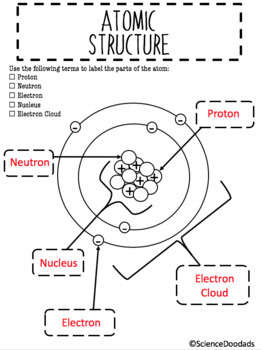
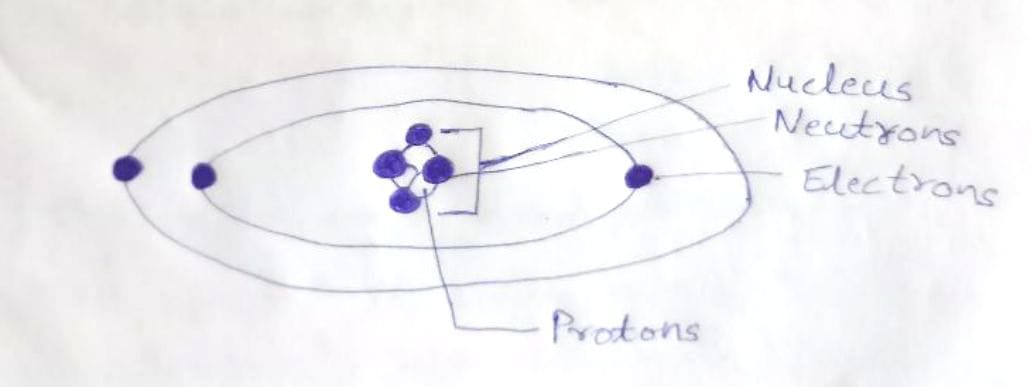
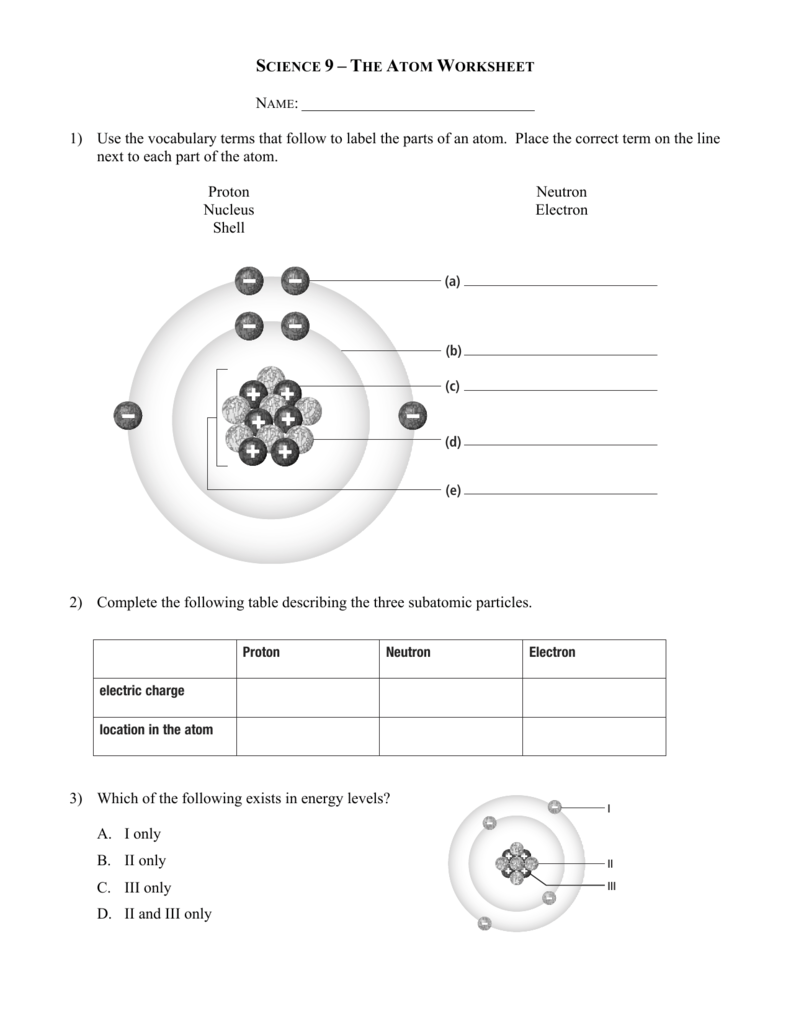
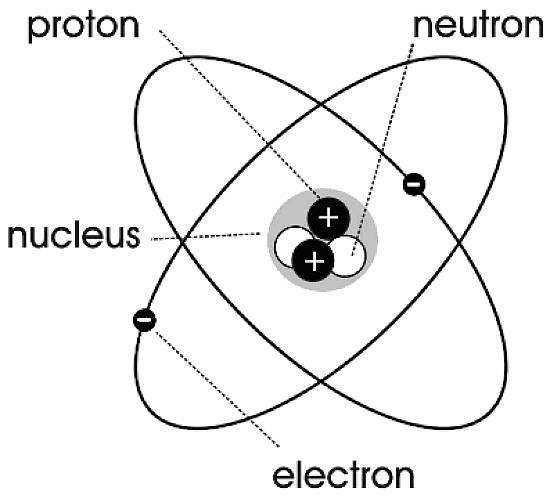
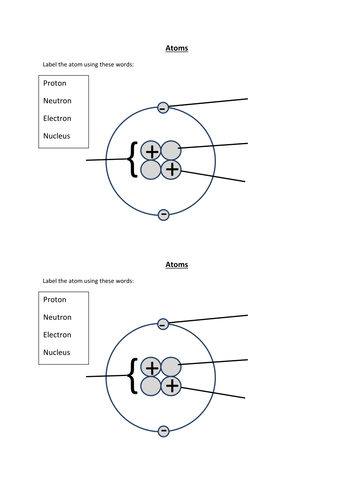
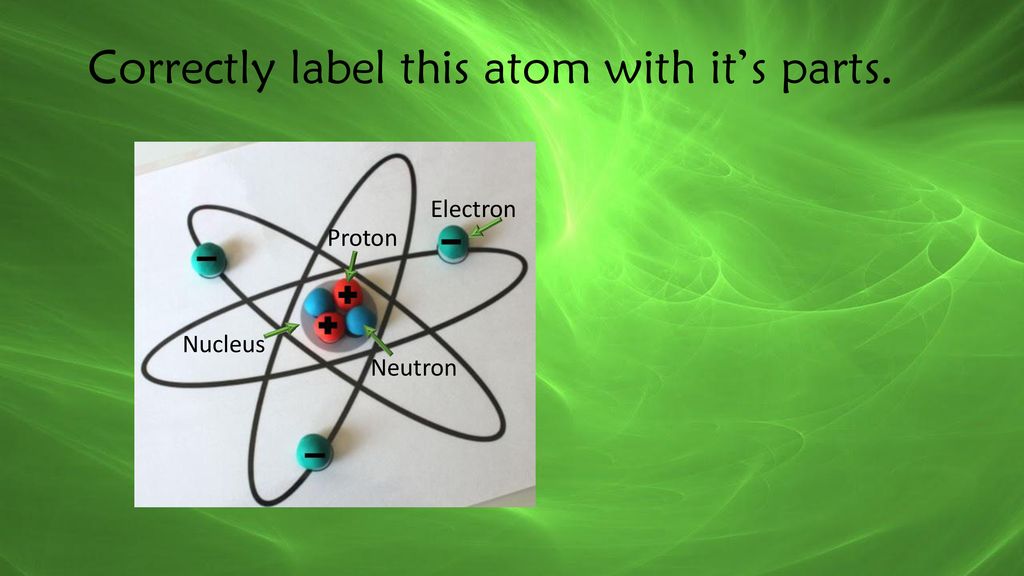
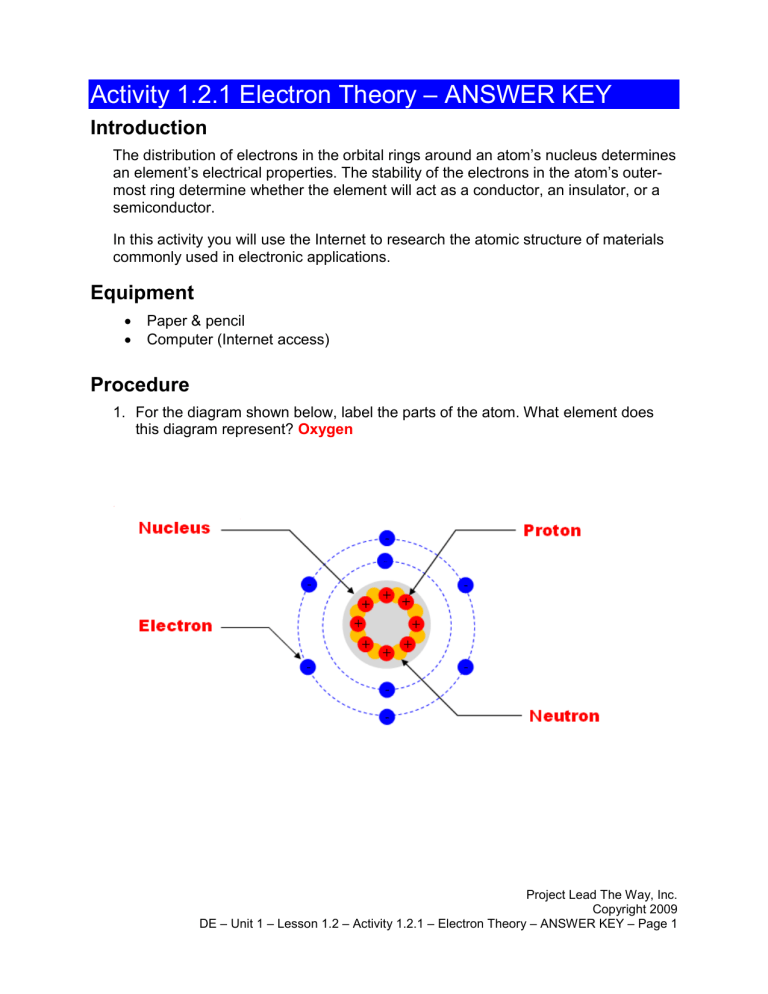
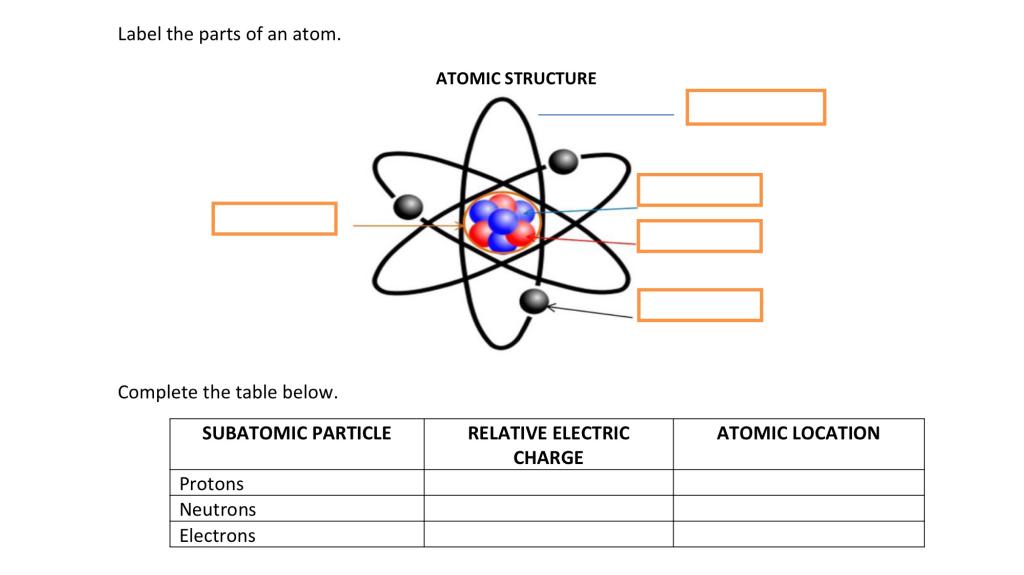
44 label the parts of the atom.
Basic Model of the Atom - Atomic Theory - ThoughtCo May 5, 2019 · The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Protons and neutrons form the atomic nucleus. Electrons are attracted to the protons in the nucleus, but are moving so quickly they fall toward it (orbit) rather than stick to protons. Label The Parts Of An Atom Teaching Resources | TPT The Parts of an Atom worksheet contains 3 pages of drawings, charts, and questions to help students understand the atom. Students define an atom, label the parts, describe and compare the protons, neutrons, and electrons, and determine the meaning of atomic number and mass number.
What Are The Parts Of An Atom? - Universe Today Dec 15, 2015 · Structure Of The Atom: Our current model of the atom can be broken down into three constituents parts – protons, neutron, and electrons. Each of these parts has an associated charge, with...

Label the parts of the atom.
Label the parts of an atom Diagram | Quizlet Label the parts of an atom 3.0 (4 reviews) + − Flashcards Learn Test Match Created by Jenny_Linde Teacher Terms in this set (5) Term electron Definition negatively charged particle of an atom found outside the nucleus Location Term neutron Definition Particle in an atom with no charge. Found in the nucleus. Location Term proton Definition Atom | Definition, Structure, History, Examples, Diagram ... Each individual atom consists of smaller particles—namely, electrons and nuclei. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. 3.4: Atomic Mass and Atomic Number - Chemistry LibreTexts Mar 3, 2021 · Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction. (3.4.1) Number of neutrons = rounded mass number − atomic number. Atoms of the element chromium ( Cr) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a ...
Label the parts of the atom.. 3.4: Atomic Mass and Atomic Number - Chemistry LibreTexts Mar 3, 2021 · Knowing the mass number and the atomic number of an atom allows you to determine the number of neutrons present in that atom by subtraction. (3.4.1) Number of neutrons = rounded mass number − atomic number. Atoms of the element chromium ( Cr) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a ... Atom | Definition, Structure, History, Examples, Diagram ... Each individual atom consists of smaller particles—namely, electrons and nuclei. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Label the parts of an atom Diagram | Quizlet Label the parts of an atom 3.0 (4 reviews) + − Flashcards Learn Test Match Created by Jenny_Linde Teacher Terms in this set (5) Term electron Definition negatively charged particle of an atom found outside the nucleus Location Term neutron Definition Particle in an atom with no charge. Found in the nucleus. Location Term proton Definition

























Komentar
Posting Komentar